Antimicrobial peptides
Antimicrobial peptides, also known as antimicrobial peptides or host defense peptides, are a class of small molecular peptides induced by organisms with broad antibacterial activity and immunomodulatory activity. They are inherent components of the body's non-specific defense system and exist in almost all life forms. Antimicrobial peptides are usually composed of 12-50 amino acids, and the relative molecular weight is about 2-5 kDa. Study found that the antibacterial peptide has rapid sterilization and broad-spectrum antibacterial activity, including gram-positive bacteria and gram-negative bacteria, multiple drug-resistant bacteria, fungi, parasites, coated viruses and tumor cells, and can in innate immunity and acquired immune before play a role of bridge, through the start-up acquired immune system to improve the body's ability to fight microbial infection. To date, more than 3,000 antimicrobial peptides have been discovered.
Based on the promotion of global no resistance policy, antimicrobial peptides have no residue, no drug resistance, no incompatibility, no withdrawal period, no toxicity and safety, etc., which has become one of the hottest topics in modern medical research. At present, there are three main production methods of antimicrobial peptides: natural extraction, chemical synthesis and bioengineering fermentation. The natural antimicrobial peptides mainly come from animals and plants, the content is very low, and the extraction process is very complicated. The chemical synthesis method is expensive, costing tens of millions of yuan per kilogram, which is not suitable for industrial production and cannot be widely used. Bioengineering fermentation is the best way to realize the industry by using fermentation technology. It has the advantages of stable quality and low cost, and is recognized by the industry as the best way to large-scale production. However, the development of commercial antimicrobial peptides is not accomplished overnight, and there are several difficulties.
01
Biological activity of antimicrobial peptides
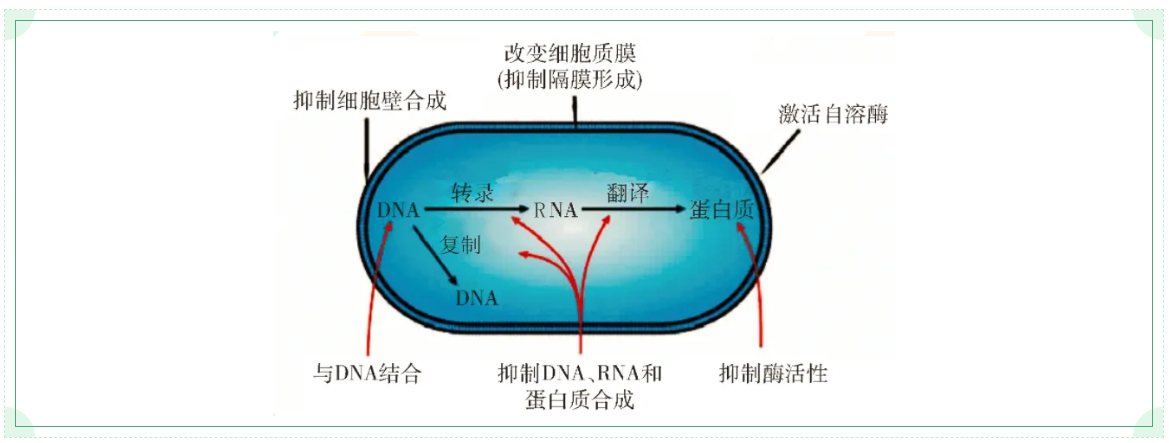
At present, there are two viewpoints about antimicrobial mechanism of antimicrobial peptides in the academic circle. One is that antimicrobial peptides act on cell membrane. The antimicrobial peptides physically act on the cell membrane of bacteria, causing the membrane to perforate and overflow the cytoplasm, thus killing the bacteria. Antimicrobial peptides have the amphiphilic properties of hydrophobic and hydrophilic, therefore the antibacterial peptide positively charged molecules and negative charge on the cell membrane phospholipid molecules formed electrostatic adsorption onto the membrane, and then the hydrophobic molecules insert in the film, so that the whole molecule absorbed into plasma membrane, it's damaged proteins and lipids in the plasma membrane of the original arrangement, form a transmembrane ion channels, lost a lot of ions, Bacteria cannot maintain the required intracellular osmotic pressure and die. The second view of bactericidal antimicrobial peptides is that they bind to targets in bacterial cells to inhibit or kill bacteria. Antimicrobial peptides can directly act on key biosynthesis processes in cells, such as inhibiting DNA replication, RNA transcription and protein synthesis, inhibiting cell wall synthesis, changing plasma membrane permeability, inhibiting synthase activity and activating bacterial autolysis, etc., such as enterobacittin inhibiting BACTERIAL RNA polymerase. There are also antimicrobial peptides that act as multichannel bactericidal agents by affecting multiple biosynthetic processes.
 In the preliminary research and development of screening antimicrobial peptides, the antimicrobial spectrum should be studied first, that is, whether the antibacterial effect is extensive and corresponding to existing clinical diseases, and then the antibacterial intensity should be studied, which is generally expressed by minimum inhibitory concentration (MIC). For example, an antimicrobial peptide has antibacterial activity against a certain pathogen, but the MIC value is too high. Even if a large dose is required to be effective, such a product is not valuable. If the MIC of enterobacitracin to Escherichia coli is <0.5ug/mL and the MIC to Salmonella is <0.1ug/mL, the product with good antibacterial effect will have market value.
In the preliminary research and development of screening antimicrobial peptides, the antimicrobial spectrum should be studied first, that is, whether the antibacterial effect is extensive and corresponding to existing clinical diseases, and then the antibacterial intensity should be studied, which is generally expressed by minimum inhibitory concentration (MIC). For example, an antimicrobial peptide has antibacterial activity against a certain pathogen, but the MIC value is too high. Even if a large dose is required to be effective, such a product is not valuable. If the MIC of enterobacitracin to Escherichia coli is <0.5ug/mL and the MIC to Salmonella is <0.1ug/mL, the product with good antibacterial effect will have market value.

02
Stability of antimicrobial peptides
Another problem in the industrial application of antimicrobial peptides is that the stability of most antimicrobial peptides is not ideal, because antimicrobial peptides, as peptides, are not particularly strong in high temperature resistance, acid and alkali resistance.
Antimicrobial peptides are biologically active peptides. In the process of biological fermentation, host bacteria mostly produce many proteases, which have degradation activity to the peptides, so that the antimicrobial peptides produced will be degraded and lost in the process of fermentation, thus losing production value. In the production process, the antimicrobial peptide material also needs to be processed from liquid to solid, which requires heat source. If an antimicrobial peptide is not heat resistant, it also loses production value.
At the same time, one of the most important ways to use antimicrobial peptides is oral administration. Antimicrobial peptides may be degraded by pepsin, trypsin, bile acid and other substances in the digestive tract, so that they cannot play their biological activities. For polypeptide products administered orally, it is very difficult.
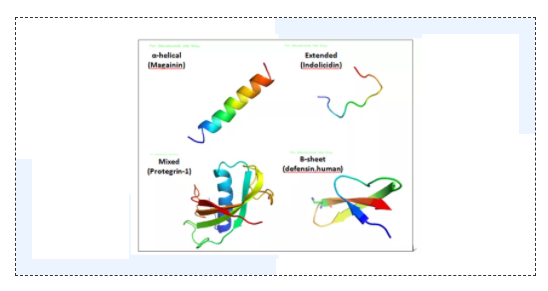
Polypeptide substance, structure determines property. For example, enterobacitracin is a locking peptide, in which the eight amino acids at the n-terminal form a circular ring structure, and 9-21 amino acids form a tail that runs through the ring, so it is super stable and has good resistance to high temperature, acid, alkali, pepsin and trypsin. Subtilisin has two disulfide bonds, and these spatial structures determine the stability of the properties. Of course, some antimicrobial peptides themselves are unstable. In order to solve this problem, researchers put forward many solutions to prevent antimicrobial peptides from being degraded in the body, such as: The amino and carboxyl ends of the peptide chain were modified by acetylation and amidation, the linear peptide chain was cyclized from the beginning to the end to improve its stability, the polyethylene glycol peptide was formed to enhance the ability of anti-hydrolase, and the antimicrobial peptide was wrapped by liposomes to make it release slowly. These programs alleviated the decrease of antimicrobial peptides to a certain extent, but the side effects were reduced yield, affected activity, more complex process, and greatly increased the production cost of antimicrobial peptides.
03
Production efficiency of antimicrobial peptides
Antimicrobial peptides have broad application prospects, but the main problem of large-scale application in pharmaceutical, food, animal husbandry and other industries is high production cost. One of the main constraints for large-scale production of antimicrobial peptides is cost, so the production efficiency directly determines whether large-scale application can be made, especially in the fields of agriculture and animal husbandry, which have strict cost requirements.
According to the above analysis, both natural extraction and chemical synthesis are not suitable for the industrialization of antimicrobial peptides, which is the only feasible method for bioengineering fermentation, but there are also many difficulties. At present, the production level of Cecropin is only 1-150mg /L reported in literature. For example, Chen et al. successfully expressed Cecropin AD in the expression system of Bacillus subtilis by using SUMo fusion expression technology, and the yield in shaker fermentation liquid was 30.6mg/L. Bi et al. successfully fused and expressed the heterozygotic peptide LFcinb-Melittin in EScherichia coli BL21, and detected the content of fermentation broth was 35mg/L. Other researchers used pichia pastoris expression system to express Avian β-defensin6 in chicken, and the content of Avian β-defensin6 in fermentation broth reached 114.9mg/L. Chinese researchers have achieved a great breakthrough in the production level of antimicrobial peptides. It is reported that the fermentation level of antimicrobial peptides in the key experimental study of antibiotic substitution technology for feeding by the Ministry of Agriculture and Rural Affairs is about 100 times that reported in literature.

04
Purification technology of antimicrobial peptides
Pure antimicrobial peptides are difficult to obtain. The separation and purification of antibacterial peptide is a key link in the process of, whether natural antibacterial peptide, or genetic engineering technology to obtain antibacterial peptide, isolation and purification of antibacterial peptides in the matrix proportion are small and there are a large number of complex proteins and other substances, and for the composition of unknown or complex antimicrobial peptide is more difficult. Therefore, the key to the separation and purification of antimicrobial peptides is not only to obtain a single peptide, but also to ensure the biological activity of the peptide, which is the basis for further scientific research. Antimicrobial peptides are a kind of small molecular peptides. Different peptides have different amino acid sequences and spatial structures, which lead to differences in physical, chemical and biological properties. A reasonable purification scheme of peptides can be designed based on the differences between peptides to be isolated and other proteins. Antimicrobial peptides account for less than 1% in the fermentation broth. It is a difficult scientific research project to obtain antimicrobial peptides with purity greater than 99.6% if 99% impurities are removed.

05
To establish the quality standard of antimicrobial peptide detection
There are two kinds of evaluation methods for antimicrobial peptides: one is to detect antimicrobial activity of antimicrobial peptides; It is mainly determined by diffusion method. The principle is that different concentrations of antibacterial peptides act on the bacteria in the culture medium, antibacterial peptides kill bacteria and thus form different sizes of antibacterial circles, according to the size of the circle, can determine the antibacterial activity of antibacterial peptides. Similarly, different concentrations of antimicrobial peptides are generally added to 0.5 Mackinel concentration of bacterial suspension, and antimicrobial peptides will affect the growth of bacterial suspension to different degrees. The strength of antibacterial activity can be judged according to the level of absorbance. The biggest problem of the detection method of antibacterial activity is that it cannot distinguish the influence of other components in microbial fermentation liquid on antibacterial results, such as culture medium and pH of fermentation liquid, etc., all have certain antibacterial activity. Even more, other bacteriostatic substances are added to the product to pass it off as antimicrobial peptides. This method of antibacterial activity detection is simple to operate and requires low personnel and equipment, which is the method adopted by most antibacterial peptide research institutes at present. However, it cannot be determined whether the product really contains antibacterial peptides, which can only indicate that the product has antibacterial activity. In the process of establishing the quality standard of antimicrobial peptides, this method can only be used as an auxiliary.
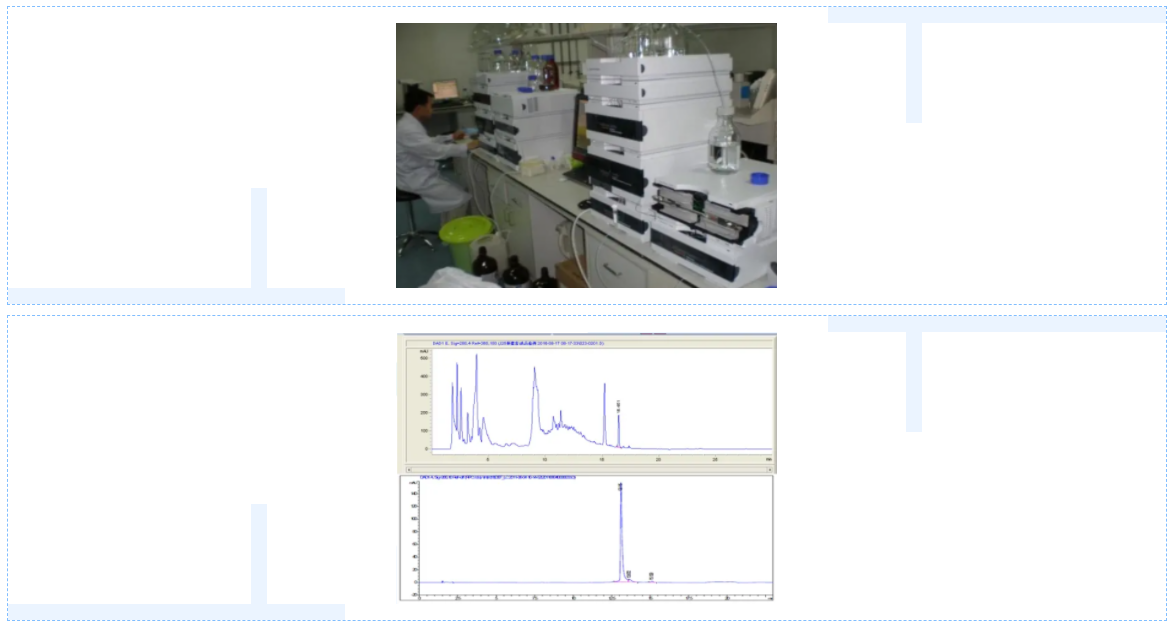
The other is qualitative and quantitative detection method, generally used is high performance liquid chromatography and capillary electrophoresis. Capillary electrophoresis equipment investment is large, the general research units use less. HPLC has strong separation ability of complex components in mixtures, and can also be used in combination with other technologies for accurate qualitative and quantitative analysis of substances. Different physical and chemical properties of antibacterial peptides can use different separation principle of chromatographic separation, such as the polarity of antibacterial peptides can be used water affinity chromatography for separation, the antibacterial peptides can be used of different charge ion exchange chromatography, but the most commonly used in the scientific research work in reverse high performance liquid chromatography (HPLC) method, is also the most scientific academia as polypeptide material testing method. For example, Li Bo studied the pilot fermentation process of bacillus subtilis host bacteria with high efficiency in secreting bombycin, and the content of bombycin reached 961.52μg/ ml by reverse high performance liquid chromatography.
06
Research and development costs are high
Antimicrobial peptides belong to polypeptide biological drugs. A new drug needs to go through a long process from the initial discovery of lead compounds to the final market. According to the qianzhan Industry Research Institute report, the cost of a new drug in the world has exceeded $1 billion and the development cycle is over 10 years. However, compared with developed countries, China's innovative drug r&d is still at a disadvantage, and there is a certain gap between China and developed countries in terms of r&d investment capacity and output. New drug research and development needs high funding demand and long investment time, which requires dual support of policy and capital.
To sum up, the development of antibacterial peptide products is faced with many difficulties, which requires a large number of researchers to overcome. With the deepening of antibacterial peptide research and the development of biotechnology, it is hoped that more and better antibacterial peptide products can be industrialized in the future, so as to promote the development of the entire pharmaceutical industry.
